By Jonathan Ugbal
No fewer than 17 staff and patients of the University of Calabar Teaching Hospital (UCTH) who were randomly selected for a rapid diagnostic test for COVID19 tested positive, says a study published recently.
That figure represents about 26 percent of the entire test population, the study says. This suggests possible community transmission as it translates to slightly more than one in four persons tested.
The State insists it has no case of COVID19 infection confirmed with the Reverse Transcriptase- Polymerase Chain Reaction (RT-PCR) method which currently is the gold standard.
But, this study obtained by CrossRiverWatch which sources say was alluded to, by the Chairman of the Nigerian Medical Association (NMA) in the state, Dr. Agam Ayuk in a recent television interview, was conducted between June 17th to 25th 2020 suggests that the low testing rate may be the reason why the state remains to the public, COVID free.
Less than 15 samples have been sent for tests to the molecular laboratories of the Nigerian Center for Disease Control (NCDC) which represents less than 0.01 percent of the over 141,500 tests carried out but the NCDC since February.
This is despite the position of the NCDC Director General, Dr. Chikee Ihekweazu that no state is currently free from COVID-19. He said this weeks after the ministerial task force from the Federal Ministry of Health which visited the state after weeks of a media face-off said there was low testing in its report.
The Study…
Antibodies test for the SARS – CoV-2, the virus that causes COVID-19 were used for this study which received ethical approval from the UCTH management.
According to the study, “Tests for antibodies to SARS-CoV-2, the virus that causes COVID-19, are more affordable, readily available, and require minimal training than current diagnostic tests.” This is because for a developing country like Nigeria, using the RT-PCR has slowed testing capacity.
The research employed a “seroepidemiological strategy,” with serological tests conducted on 66 volunteering staff and patients at the UCTH “to determine the extent of exposure to SARS-CoV-2, from 17th to 25th June 2020.
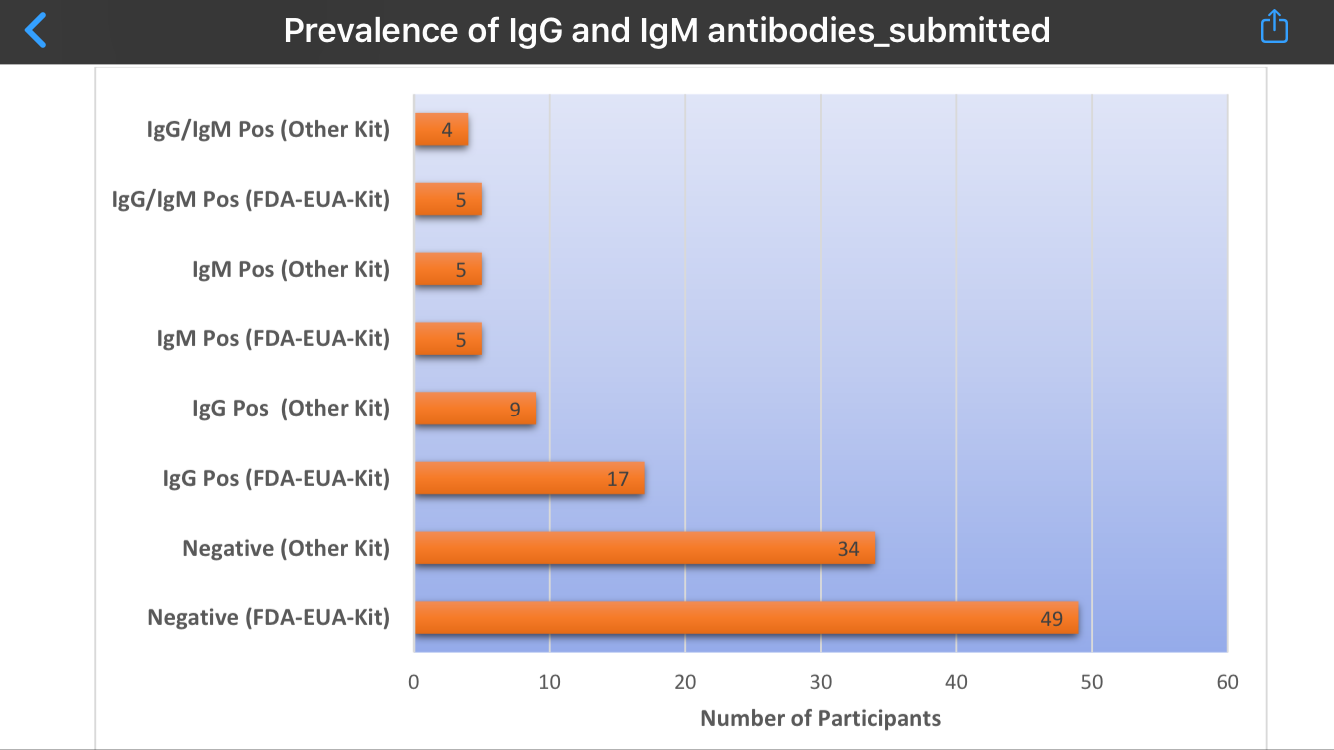
“Using a COVID-19 IgG/IgM Rapid Test Cassette with emergency use authorization (EUA) from the Food and Drug Administration (FDA) of the United States, it was observed that of the 66 samples tested, 5 (7.6%) were both IgG and IgM positive and 17 (26%) were IgG positive. Moreover, for 44 of the 66 participants, simultaneous tests were carried out using a rapid test kit from a different manufacturer but without FDA-EUA and all the results completely matched with the FDA-EUA kit, except one case where the FDA-EUA kit showed positive for both IgG and IgM while the other kit was positive only for IgM.
“The 26% positive IgG indicates a high exposure rate for the hospital staff and patients and points to community transmission where the facility is situated. Hence, immediate activation of WHO guidelines for controlling community transmission is called for. These results can further serve as a pilot study to guide public health policies in response to COVID-19 pandemic in both the general population and in healthcare settings.”
What is IgG and IgM?
IgG and IgM are immunoglobulins – protein substances that are produced in the body to fight infection. Each infection has specific antibodies.
Now, the IgG test detects IgG antibodies that develop in most patients within seven to 10 days after symptoms of COVID-19 begin. These antibodies remain in the blood even after an infection has passed and indicates that you have had COVID-19 in the recent past and have developed antibodies that may protect you from future infection even though it remains unclear whether this protection antibodies might help against reinfection.
For the IgM antibodies, these are usually the first antibody produced by the immune system when a virus attacks. A positive IgM test shows that you may have been infected and that your immune system has started responding to the virus. It also means you may still be infected, or you may have recently recovered from a COVID-19 infection.
Back to the Study…
The study which is been conducted across different states of the federation had volunteers aged 18 and above only with 38 males representing 58 percent and 28 females representing 42 percent.
The kits used include the COVID-19 IgG/IgM Rapid Test Cassette (Whole Blood/Serum/Plasma) which received emergency use authorization (EUA) from the FDA (EUA200056 Healgen LOA 05-29- 2020) for “use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection”.
Denoted as FDA-EUA-Kit in the report, it was purchased from Confirm Biosciences (10123 Carroll Canyon Road, San Diego, CA 92131), the sole distributor of Healgen products in North America.
Simultaneously with the FDA-EUA-Kit, another kit (manufactured by Zhejiang Orient Gene Biotech) used in 44 of the 66 total participants, was donated to UCTH by Lafarge Africa Plc. This kit was denoted “Other Kit”, in the report.
“Of the 66 participants, 62 (94%) had resided in Calabar Municipality or Calabar South Local Government Area of Cross River State for at least 12 months prior to the testing,” the report of the study said.
The Results of the Study…

The FDA-EUA Kit test results for 5 of the 66 participants were both IgG and IgM positive (7.6%), 17 of the 66 were IgG positive (26%) while 49 (74%) were negative. There was no inconclusive result. For 44 of these 66 participants, simultaneous testing with the other kit yielded results that completely matched except one case where the FDA-EUA kit showed positive for both IgG and IgM while the other kit was positive only for IgM. Even for this one case, since the detection of IgM has the same implication as the detection of IgG and IgM, namely, acute or recent infection, the two test kits produced the same results.
“For the 44 participants tested with the ‘Other Kit’ in addition to the FDA-EUA Kit, there were 4 IgG and IgM positive and 9 IgG positive. These results provide evidence of possible community transmission of COVID- 19 in UCTH and Calabar, since 62 (94%) of participants had lived in Calabar for at least 12 months before the research.
“The work here serves as a pilot study to guide public health policies in response to COVID-19 pandemic both in the general population and in healthcare settings.”
Prevalence of IgG and IgM antibodies_submitted
And To The Politicking…
There have been accusations and counter accusations from medical associations and the state government about the level of testing in the state.
The Governor, Benedict Ayade had once talked down on the RT-PCR method and insisted on the use of antibodies as well as their extraction for the development of vaccines.
But, following a leak health advisory from the UCTH, wherein a test on the remains of the father of the Deputy Chairman, Medical Advisory Committee returned positive, the health commissioner, Betta Edu said that rapid tests were unreliable as index cases could only be confirmed with RT-PCR.

The fears increased after the NMA claimed that the state had not sent any sample for test despite announcing that the ones sent returned negative. The state responded and claimed that some medics were spreading fear and patients were dying due to negligence of duty.
In May, Taiwo Adebulu of Cable Newspapers who disguised as someone with travel history had sneaked into Cross River and weeks after his sample was reluctantly taken, the result is yet to be announced, suggesting that not all samples taken were sent to NCDC certified laboratories for analysis.
But, Mr. Ayade on Wednesday said those insinuating that the state was hiding its COVID-19 status were been mischievous.
“We introduced the use of nose masks early. In fact, we were the first to lock our borders, we introduced face shields. We were the first in anything that were required as defensive mechanism against COVID-19. So, it became obvious and clear that we were able to contain with other airborne related diseases. So, we have no reason to suggest that there is pandemic in Cross River. Statistics also show it,” Ayade said in a dance and cocktail party to signal the end of phase one of the management of the pandemic in the country.
He added that: “It is a pandemic, therefore, when you say somebody has Coronavirus virus, it means it can spread to all family members, they will all come down with it. You can’t hide it. Mischief makers are wondering why Cross River which is surrounded by COVID-19 infested states and country is free from it but they forget that Coronavirus is not politics, it is not a political issue but a biological issue and therefore, can be contained, can be dealt it.”

He told residents to be more scared now than before. “Now, I have no control.I have officially handed over Cross River to the federal government control as far as COVID-19 is concerned because I can’t close my borders anymore, I don’t have control over the airspace. So, whatever happens going forward, I wish to say kudos to Cross River and Cross Riverians for being a state and a people that walked through this big challenge and ended up victorious,” he said.
On the low testing rate of the state, the governor said: “We are calling on the NCDC and the federal Ministry of Health to set up a COVID-19 testing centre in Cross River state. Once we have a testing centre that has been certified by the NCDC we will be encouraged to do more testing because the rapid testing method is not acceptable and reliable.”
The health commissioner had once said it costs the state N200,000 which is four times more than the national average to transport a sample to be tested.
Since You Are Here, Support Good JournalismCrossRiverWatch was founded on the ideals of deploying tech tools to report in an ethical manner, news, views and analysis with a narrative that ensures transparency in governance, a good society and an accountable democracy. Everyone appreciates good journalism but it costs a lot of money. Nonetheless, it cannot be sacrificed on the altar of news commercialization. Consider making a modest contribution to support CrossRiverWatch's journalism of credibility and integrity in order to ensure that all have continuous free access to our noble endeavor. CLICK HERE |
New Feature: Don't miss any of our news again.Get all our articles in your facebook chat box.Click the Facebook Messenger Icon below to subscribe now
Text Advert by CRWatch :Place Yours

Will You To Learn How To Make Millions Of Naira Making Special Creams From Your Kitchen?.Click Here
Expose Your Business And Make More Sales. Advertise On CrossRiverWatch.com Today


Leave feedback about this